Key points
- Clinics around Australia have experienced more people enquiring about clinical trials since COVID-19.
- Experts say people now have a genuine interest in the science behind trials or in "contributing to something".
- The trials are worth big money, with the government offering R&D tax incentives to biotech and pharmaceutical companies.
For Diane Morrison, it was her son's work in clinical research that inspired her to get involved in clinical trials.
The 61-year-old, who lives on Sydney's Northern Beaches, has taken part in three clinical trials in as many years. Her current trial is for a potential vaccine for respiratory syncytial virus (RSV), which causes infections of the lungs and respiratory tract and can be fatal, especially in babies and the elderly.
"Clinical researchers work so hard to find a cure for people's illnesses. I thought if it saves one person's life, it's going to be worth it," she told SBS News.
While there may once have been a stigma around being a human guinea pig, Ms Morrison says people's thoughts around clinical trials seem to have completely shifted thanks to COVID-19.
"We were all hanging out for that vaccine. Everyone was aware of COVID, because everyone could catch it. So it definitely made people more aware of clinical research and how they can be involved, which is great."
To date, Ms Morrison has never had any adverse reactions from the trials she's done and she says she's felt well cared for.
"If I've had any changes in symptoms, the clinic is straight onto it. When I got COVID in June, they were ringing me all the time. They've been truly amazing," she said.

Sydney-based Diane Morrison says if her participation in clinical trials helps to save one person's life, "it's worth it". Source: Supplied
'An interest in contributing to something'
Clinics around Australia have experienced heightened interest in trials as a result of COVID-19, with the enthusiasm continuing.
Evrima Technologies, which helps facilitate clinical trials, has seen a more than 70 per cent jump in people registering their interest from the fourth quarter of 2021 to the third quarter of 2022.
"In the last couple of years, interest in trial opportunities has certainly increased across all age groups," Charlotte Bradshaw, Evrima's CEO and founder said.
"Prior to the pandemic, we just took it for granted that we could get a prescription for medicine without giving too much thought to how it came to be.
"Now, because of the research around vaccines and other treatments for COVID, people have an interest in what it takes to get a new treatment, a new device or a new therapy onto the market that's available for everyone who needs it."
Jeff Wall, director of Northern Beaches Clinical Research, agrees awareness has certainly grown among the community since the pandemic.
"Because clinical trials have been in the mainstream media, there's been a lot more talk about the benefit of having them and moving them through," he said.
"People have a genuine interest either in the science behind the trial or in contributing to something, and that's a great thing."
How clinical trials work
To prove a treatment is safe and effective enough to bring to market, it must first undergo pre-clinical trials in different animals at high doses before undergoing four phases of human trials — at much lower doses.
Phase 1 trials examine a drug's potential toxicity, with participants required to be in good health. They typically attract younger people who are prepared to give up significant amounts of time under observation in exchange for remuneration of up to $6,000.
Participants of later trials, when the drug has been proven safe in humans, tend to be those with a condition that would benefit from the treatment, or healthy people wanting to assist future generations. These trials typically require regular clinic visits with expenses such as lunch, parking, and care covered.
All participants are given lots of information about potential benefits and risks, and can pull out of trials at any time.
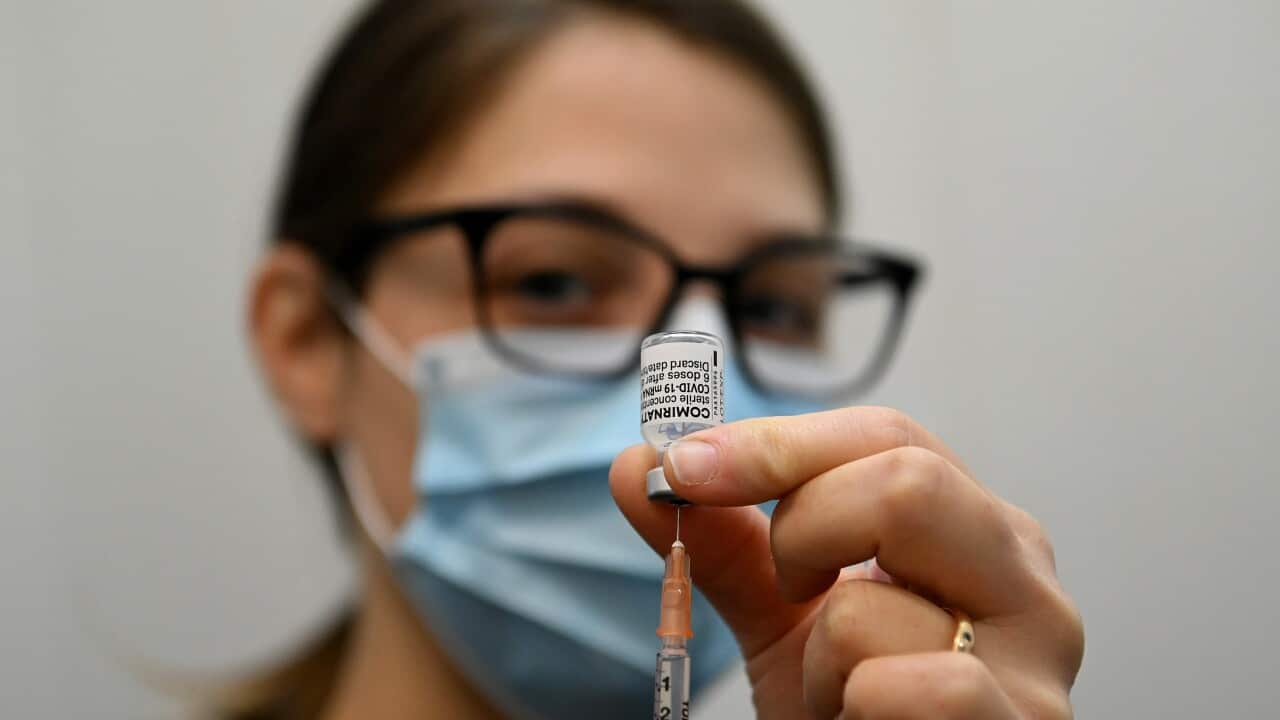
Particular interest in COVID-19 vaccine trials
For Phase 1 clinics, COVID-19 was a "double-edged sword," says Sam Dowd, innovation manager at Linear Research, a purpose-built trials facility in Perth.
"Pre-COVID, our phase 1 trials largely attracted backpackers and international students. So during COVID there were less of these groups because there was no international travel.
"But all these new participants started coming for more altruistic reasons, which made up for that," he added.
"We really saw a boost, and this interest is continuing."
There was particular interest in Linear's phase 1 COVID-19 vaccine trial, with Timothy Roberts, 62, one to put his hand up.
Like many Australians, what the Perth resident found hardest about COVID-19 was a "sensation of helplessness".
Not only was he locked down, but his elderly father was fragile, and among his three sons, one was a frontline worker, another was in hospitality and one was a university student, suddenly confined to study in his bedroom.
When he saw a TV ad to participate in a COVID-19 vaccine trial, he didn't hesitate.

When Timothy Roberts felt "helpless" during the pandemic, signing up to participate in a phase 1 COVID-19 vaccine trial felt like something tangible he could do to help. Source: Supplied
The trial lasted more than a year and included regular clinic appointments and follow-up.
"I knew that COVID vaccines had to come from somewhere. And at a time when I had no control, I was doing something tangible and that felt good."
Trials are big business
This new eagerness among Australians to participate in clinical trials is music to the ears of the clinical research industry and the Australian government.
Globally, the industry is growing fast thanks to the emergence of new treatments and new technology, such as mRNA vaccines, which all require data to be approved.
The Australian government wants as many biotech and pharmaceutical companies as possible to conduct their trials here. To entice them, it offers a high-quality regulatory system and human research ethics governance framework, as well as tax incentives for research and development.
There's a huge payoff; clinical trials contribute an estimated $1.1 billion a year to the national economy. Not only do they create jobs and boost Australia's reputation, but any Australian-made breakthroughs in health and medical innovation lead to improved health outcomes and new sources of economic growth.
The lures appear to be working.
There were 1,957 trials registered in Australia in 2021, up from 1,756 in 2020, 827 in 2010 and just 51 in 2000, according to the Australian New Zealand Clinical Trials Registry.
So far in 2022, there have been 1,652 trials registered.

Clinical trials contribute an estimated $1.1 billion a year to the Australian economy. Source: SBS News
"It's pulling research into the industry, so it's creating more jobs. And it's just the ability for us to be known as a destination to conduct high-quality research," she said.
This high volume of trials explains why Australians may be seeing more ads to participate in trials on their social media feeds and in traditional media, she said.
GPs are also playing a more active role in referring people to trials that may be relevant to their condition, or if their needs are not being met by conventional treatments.
Bigger, more diverse trials
While trials are increasing in volume, they are also increasing in diversity.
Ms Bradshaw said most participants now are interested in trials around chronic lifestyle diseases and vaccine research.
There is also a push to involve more people in remote and regional areas, with technology now making decentralised trials possible.
"One thing that the industry is really focused on at the moment is getting access to clinical trials for people in regional and remote communities," she added.
"It's a fantastic opportunity to open up the conversation both within families and the community, to talk about participating in research."
Conducting trials remotely not only expands the diversity of the participant pool, but also provides access to possible treatments for people with rare diseases who live outside major cities.
Mr Dowd says this creates new opportunities for treatment in the community.
"Bringing these treatments through trials to people in more rural areas who otherwise wouldn't have access to them, especially in phases 2 and 3, is quite exciting," he said.
Ms Morrison says she has come to understand that "for some people, their life depends on these trials".
"If none of us did clinical trials, these drugs wouldn't get anywhere," she said.
"I try to encourage more people to do it because I think the more people participate, the more we're going to get rid of these horrible illnesses and diseases."